Abstract
Introduction: Heparin induced thrombocytopenia (HIT) and APS (antiphospholipid syndrome) are acquired thrombophilia's characterized by both arterial and venous thromboembolic events (TE). As a common feature APS and HIT share the presence of pathological antibodies, which play a crucial role in disease pathogenesis. In HIT, antibodies are directed to platelet factor (PF) 4 and heparin, respectively, and in APS, antibodies target anti-phospholipids (anti-cardiolipin antibody, anti-Beta2- glycoprotein I and lupus anticoagulant).
There is growing evidence that complement- and neutrophil activation in the form of neutrophil extracellular traps (NETs) play an important role in the pathogenesis of thrombosis, the so-called "immunothrombosis". Insufficient control with subsequent unrestrained complement activation results in thrombosis as seen in patients with paroxysmal nocturnal hemoglobinuria (PNH). Therapeutic inhibition of complement activation abrogates thrombosis in these patients, emphasizing the important role of complement activation in the pathogenesis of thrombosis. It has been demonstrated, that complement activation products efficiently induce neutrophil activation, and therapeutic complement inhibition in sepsis and PNH prevents neutrophil activation. Here, we investigate the role of complement activation and neutrophil activation in patients with APS and HIT, respectively.
Methods To assess the association of complement activation and NET Formation, we measured complement activation products (C4bc, C3bc) and marks for neutrophil activation, such as nucleosomes and human neutrophil elastase-α1- antitrypsin complexes (HNE) by ELISA in plasma of 34 primary APS- and 26 HIT- patients and 31 healthy controls. Groups have been compared using the Mann- Whitney-Rank Test. Values are indicated as median and range, p<0.05 is considered statistically significant.
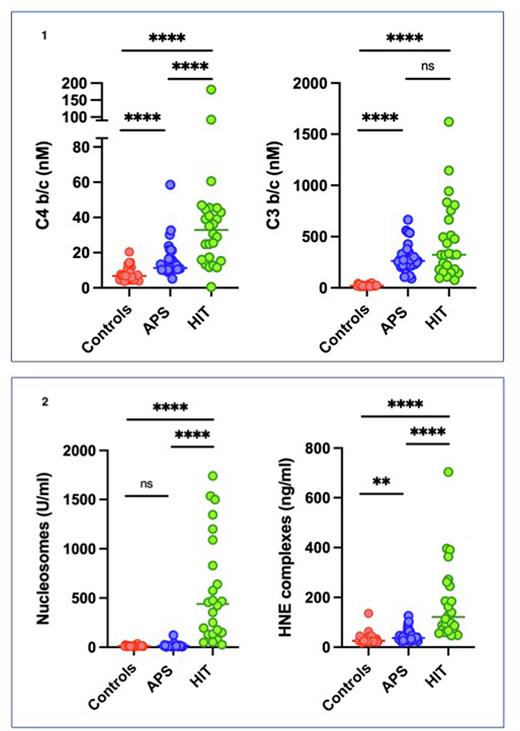
Results Patients with APS and HIT showed sign. higher levels of complement activation products as compared to healthy controls. Complement activation mainly of the classical (C4b/c) and alternate pathway (C3b/c) in APS (11 nM and 263 nM) and in HIT (33 nM and 324 nM) were sign. higher (p<0.0001) as compared to the healthy controls (7 nM and 19 nM). C4bc levels were sign. higher in HIT as compared to APS patients (p<0001), but there was no difference in C3b/c levels between these two groups (see figure 1). Patients with HIT had a sig. (p<0.0001) higher nucleosomes (440 U/ml) and HNE (121 ng/ml) as compared to healthy controls (12 U/ml and 26 ng/ml). HNE levels were sign. (p<0.001) higher in APS patients (37 ng/ml) as compared to healthy controls (26 ng/ml), however no difference was observed in nucleosome levels (12 U/ml vs 14 U/ml) (see figure 2).
Based on the number of positive antibodies, eleven APS patients have been stratified as high-risk and 24 APS patients as low- to intermediate risk. C4bc levels were sign. increased in the high-risk patients as compared to the low- to intermediate risk group, whereas no difference in C3b/c levels could be observed between the 2 groups. Similarly, HNE complexes sign. increased in the high-risk patients as compared to the low- to intermediate risk group, whereas no difference in nucleosome levels could be observed between the 2 groups.
Six HIT patients (22%) had a diagnosed TE event whereas 17 patients (63%) had no diagnosed TE. In four patients (15%) no clinical data were available, and they have been excluded from subgroup analysis. Interestingly, the measured markers for complement - and neutrophil activation in HIT patient with and without thrombosis were not different.
Conclusions We demonstrate complement activation in patients with HIT and APS. Based on the simultaneous raise in nucleosomes and HNE, there is evidence of NET formation in HIT, whereas only weak neutrophil activation can be found in APS. In APS classical complement activation and neutrophil activation correlates with APS risk stratification. Our results demonstrate classical complement pathway- and neutrophil activation in HIT and APS patients. These findings may form the base for new therapeutic strategies targeting complement and neutrophil activation.
Disclosures
Angelillo-Scherrer:SNSF: Research Funding; Vaderis: Consultancy; Pfizer: Other: Speaker fees; Silence Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.